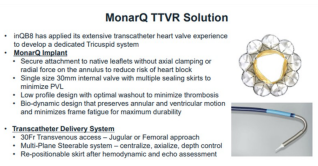
Since the first implantation in November 2023, a total of 9 patients have been enrolled in MonarQ™ TTVR system's global FIM study. Among them, there are 2 cases in Denmark, 2 in Sweden, 4 in the United States, and 1 in Canada. Follow-up results show that all 9 patients have recovered well postoperatively, with no deaths or strokes or other MACE events occurring. There have been no new pacemaker implants, and tricuspid regurgitation has been significantly improved after discharge for all patients. One patient, due to annulus size, had poor stability of the interventional valve and subsequently underwent surgical intervention.
The ongoing enrollment in FIM study of MonarQ™ confirms the feasibility and safety of this TTVR system.