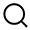
2025.05.24
EuroPCR 2025 One-Year Follow-Up Results from Pivotal Trial of TaurusTrioTM ···
Highlights: The pivotal clinical trial enrolled 116 symptomatic patients wi···
2025.05.23
EuroPCR 2025 30-Day Follow-Up Results from Initial Feasibility Study of Hig···
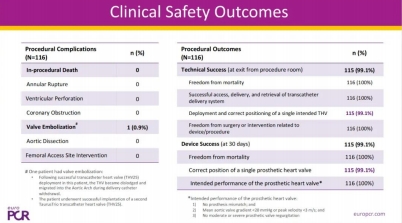
Highlights: 1. The reported results were derived from a single-center feasi···
2025.05.23
EuroPCR 2025 One-Year Follow-Up Results from Pivotal Trial of GeminiOne® TE···
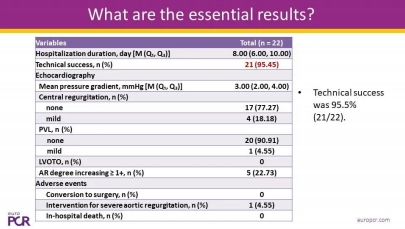
Highlights: The pivotal trial enrolled 130 patients (including 10 roll-in p···

2025.04.21
ONE-YEAR FOLLOW-UP RESULTS OF THE REGISTRATION TRIAL OF TAURUSTRIO™ TAV SY···
On April 18, 2025, at the China Valve (Hangzhou) 2025 conference, Prof. Yan Yunf···

2025.04.15
REGISTRATION APPLICATION FOR TaurusTrio™ TAV SYSTEM ACCEPTED BY THE NMPA
On April 14, 2025, Peijia Medical announced that the NMPA has formally accepted ···

2025.04.08
HighLife granted US FDA Breakthrough Device Designation for its TMVR soluti···
We are thrilled to congratulate our incredible partner HighLife Medical on ···
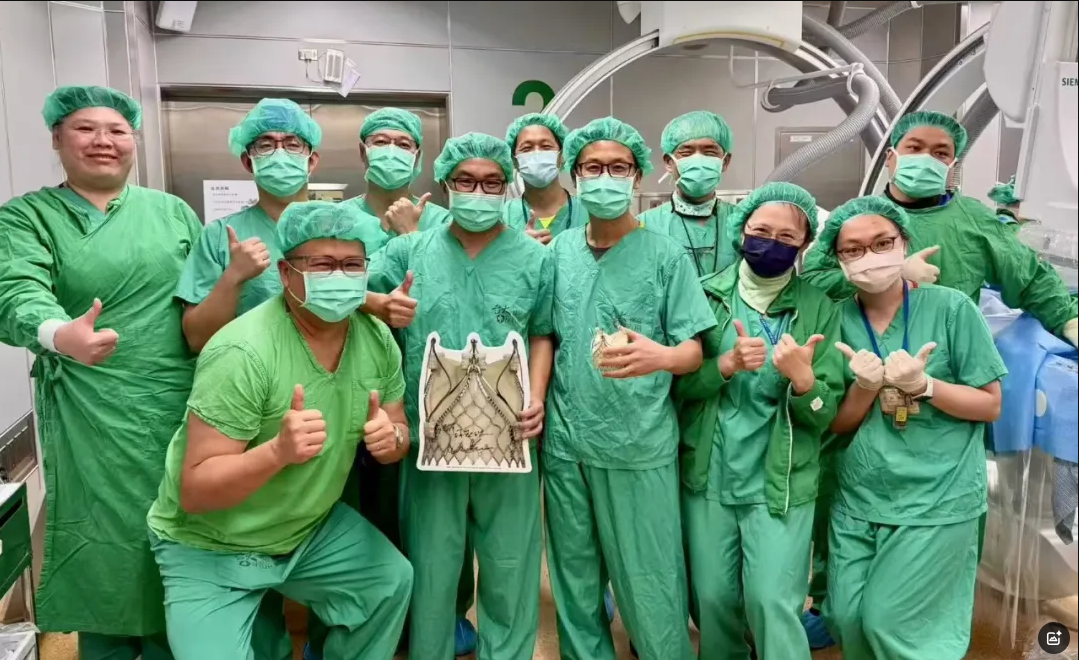
2025.03.05
SUCCESSFULLY COMPLETION OF THE FIRST TWO IMPLANTSWITH THE TrilogyTM TRANSCA···
This announcement is made by Peijia Medical Limited (the “ Company”, together wi···

2025.02.10
PROFESSOR GE JUNBO'S TEAM SUCCESSFULLY COMPLETED THE FIRST NATIONAL IMPLAN···
The team led by Academician Ge Junbo from Zhongshan Hospitalaffiliated with Fuda···
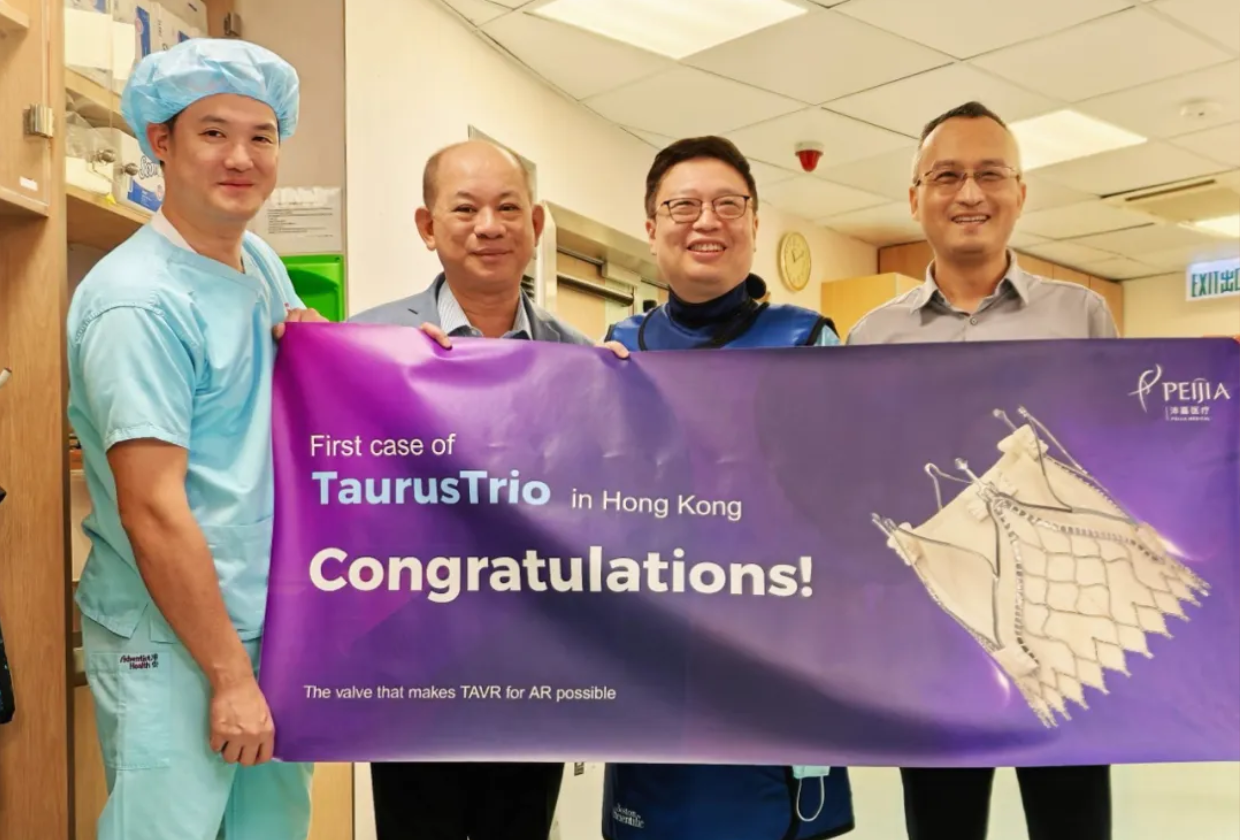
2024.11.28
TaurusTrio™ TAVR SYSTEM (TaurusTrio™) SUCCESSFULLYCOMPLETED THE FIRST FEE-B···
In November 2024, Peijia Medical successfully implanted the TaurusTrio™, a self-···
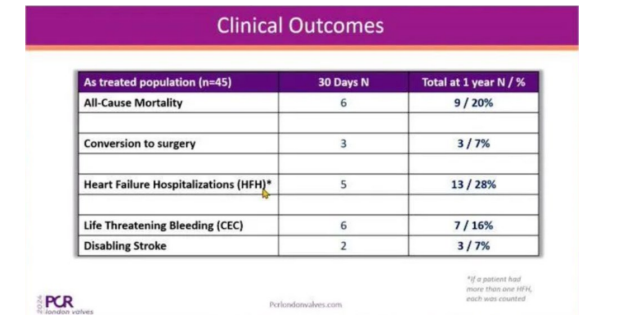
2024.11.26
PCR London Valves : HighLife® TSMVR SYSTEM (HighLife®) ANNOUNCED THE 1- YEA···
According to the clinical results, HighLife® achieved atechnical success rate of···
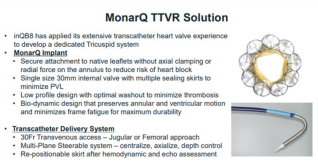
2024.11.06
THE LATEST PROGRESS IN THE FIM STUDY OF MonarQ™ IN EUROPE AND AMERICA WAS A···
Since the first implantation in November 2023, a total
of 9 patients have been ···

2024.11.05
TWO-YEAR FOLLOW-UP RESULTS OF HighLife® FEASIBILITY STUDY WERE ANNOUNCED AT···
Two-year clinical data of the HighLife® TSMVR
technology's feasibility tria···